Bifunctional catalysis
We are interested in the design of air/moisture insensitive catalysts capable of activating both the nucleophilic and electrophilic reaction partners simultaneously and controlling their encounter in a chiral environment. Such strategies allow one to aspire towards the control over complex processes using multiple weak catalyst-substrate interactions under mild conditions.
'Enantioselective alkylative kinetic resolution of 2‑oxindole-derived enolates promoted by bifunctional phase transfer catalysts'
E. Sorrentino and S. J. Connon*, Org. Lett. 2016, 18, 5204.
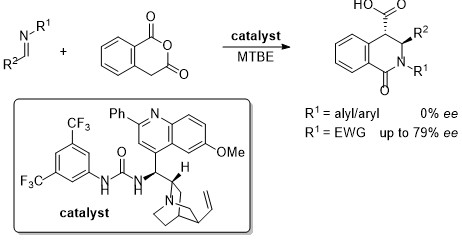
'The first catalytic asymmetric cycloadditions of imines with an enolisable anhydride'
S. A. Cronin, A. Gutiérrez Collar, S. Gundala, C. Cornaggia, E. Torrente, F. Manoni, A. Botte, B. Twamley and S. J. Connon* Org. Biomol. Chem. 2016, 14, 6955.
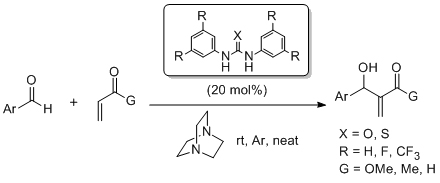
'Catalytic formal cycloadditions between anhydrides and ketones: excellent enantio and diastereocontrol, controllable decarboxylation and the formation of adjacent quaternary stereocentres'
C. Cornaggia, S. Gundala, F. Manoni, N. Gopalasetty and S. J. Connon*, Org. Biomol. Chem., 2016, 14, 3040.
This article was awarded Hot-paper status by referees.
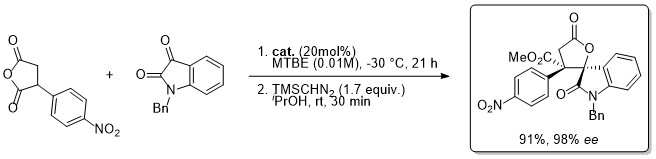
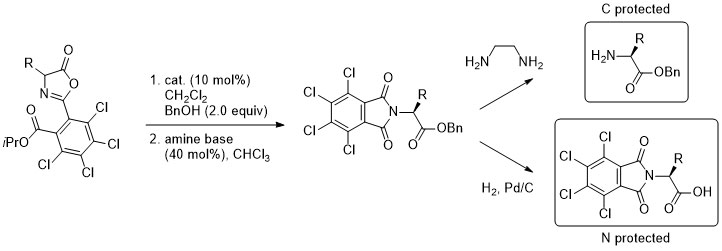
'A practical aryl unit for azlactone dynamic kinetic resolution: orthogonally protected products and a ligation-inspired coupling process'
S. Tallon, F. Manoni and S. J. Connon*, Angew. Chem. Int. Ed. 2015, 54, 813.
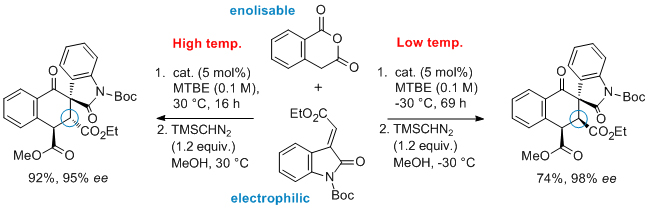
'Catalytic asymmetric Tamura cycloadditions'
F. Manoni and S. J. Connon*, Angew. Chem. Int. Ed. 2014, 53, 2628.
Highlighted in Synfacts (2014, 423)
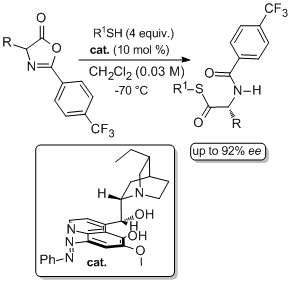
'C-5’ Substituted cinchona alkaloid derivatives catalyze the first highly enantioselective dynamic kinetic resolutions of azlactones by thiolysis'
C. Palacio and S. J. Connon*, Eur. J. Org. Chem. 2013, 5398.
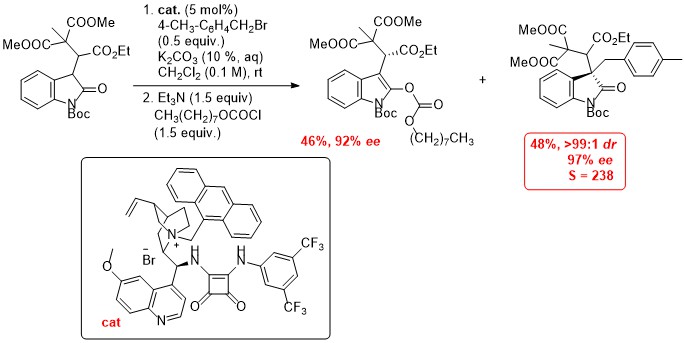
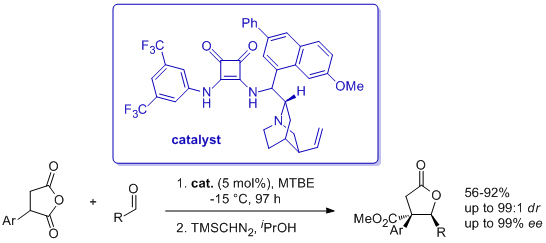
'Catalytic, enantio- and diastereoselective synthesis of γ-butyrolactones incorporating quaternary stereocentres'
F. Manoni, C. Cornaggia, J. Murray, S. Tallon and S. J. Connon*, Chem. Commun. 2012, 48, 6502.
'A catalytic asymmetric reaction involving enolizable anhydrides'
C. Cornaggia, F. Manoni, E. Torrente, S. Tallon and S. J. Connon*, Org. Lett. 2012, 14, 1850.
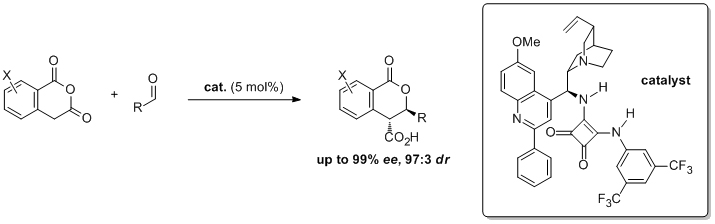
'The dynamic kinetic resolution of azlactones with thiol nucleophiles catalysed by arylated, deoxygenated cinchona alkaloids'
Z. Rodriguez-Docampo, C. Quigley, S. Tallon, S. J. Connon*, J. Org. Chem. 2012, 77, 2407.
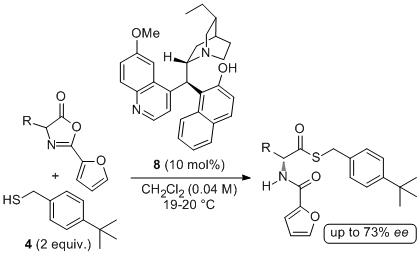
'A novel C-5' substituted cinchona alkaloid-derived catalyst promotes additions of alkyl thiols to nitroolefins with excellent enantioselectivity'
C. Palacio and S. J. Connon*, Chem. Commun. 2012, 48, 2849.
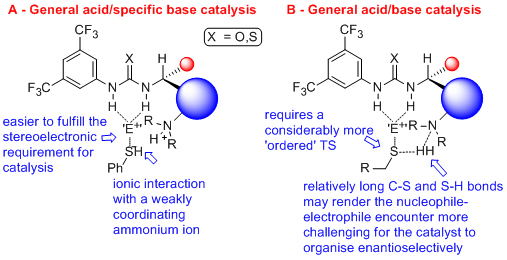
'Organocatalytic asymmetric additions to meso-anhydrides and azlactones'
Z. Rodriguez Docampo and S. J. Connon*, ChemCatChem 2012, 4, 151.
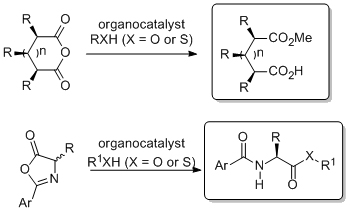
'Highly tunable arylated cinchona alkaloids as bifunctional catalysts'
C. Quigley, Z. Rodríguez Docampo and S. J. Connon*, Chem. Commun. 2012, 48, 1443.
6th Most accessed paper in Chemical Communications – September 2011
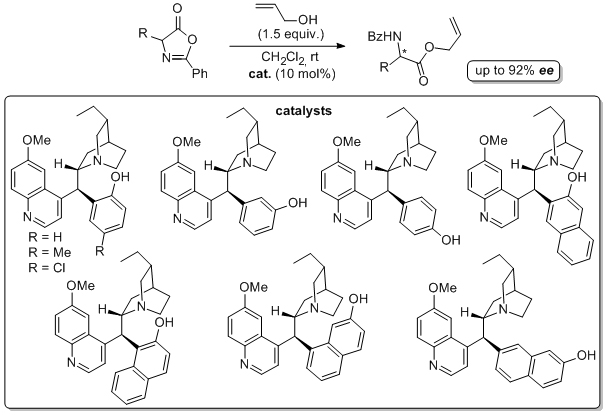
'A new class of urea-substituted cinchona alkaloid promote highly enantioselective nitroaldol reactions of trifluoromethylketones'
C. Palacio and S. J. Connon*, Org. Lett. 2011, 13, 1298.
Highlighted in Synfacts (2011, 559)
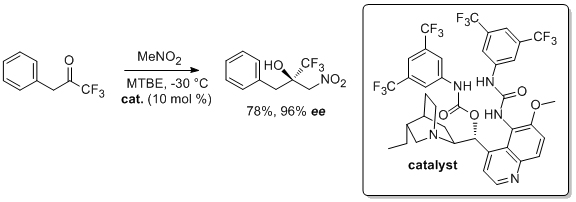
'Urea-catalyzed transthioesterification: towards a new kinetic resolution methodology'
S. Tallon, A. C. Lawlor and S. J. Connon*, Arkivoc 2011, (iv), 115.
Special issue in honour of Prof. Dr. S. Blechert
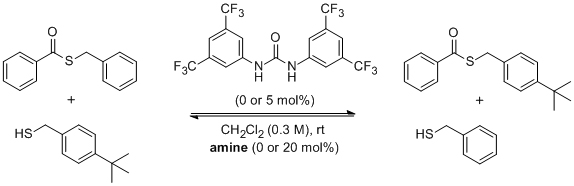
'Synergistic organocatalysis in the kinetic resolution of secondary thiols with concomitant desymmetrisation of an anhydride'
A. Peschiulli, B. Procuranti, C. J. O'Connor and S. J. Connon*, Nature Chemistry 2010, 2, 380. Highlighted in Synfacts (2010, 925)
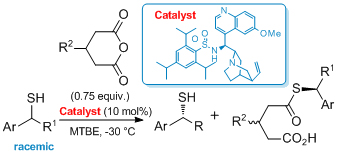
'The design of novel, synthetically useful (thio)urea-based organocatalysts'
S. J. Connon*, Synlett 2009, 354.
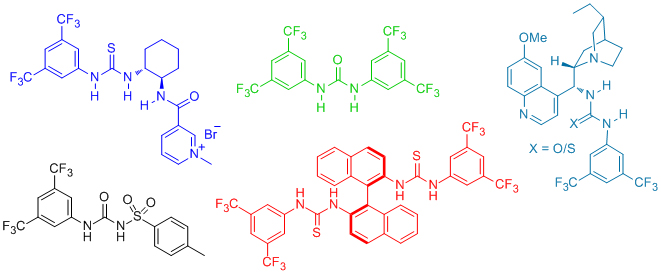
'Organocatalytic asymmetric addition of alcohols and thiols to activated electrophiles–efficient dynamic kinetic resolution and desymmetrization protocols'
A. Peschiulli, C. Quigley, S. Tallon, Y. K. Gun'ko* and S. J. Connon*, J. Org. Chem. 2008, 73, 6409.
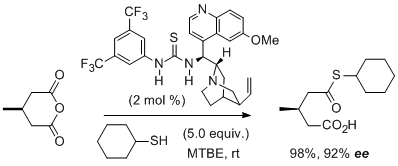
'Highly enantioselective desymmetrization of meso anhydrides by a bifunctional thiourea-based organocatalyst at low catalyst loadings and room temperature'
A. Peschiulli, Y. Gun'ko* and S. J. Connon*, J. Org. Chem. 2008, 73, 2454.
Highlighted in Synfacts (2008, 529)
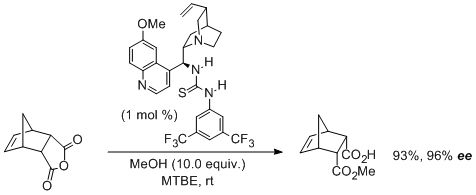
'Asymmetric catalysis with bifunctional cinchona alkaloid-based urea- and thiourea organocatalysts'
S. J. Connon*, Chem. Commun. 2008, 2499.
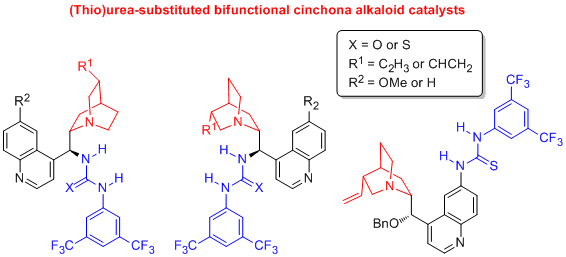
'Readily accessible 9-epi-amino cinchona alkaloid derivatives promote efficient, highly enantioselective additions of aldehydes and ketones to nitroolefins'
S. H. McCooey and S. J. Connon*, Org. Lett. 2007, 9, 599.
In the top 20 (No. 15) most cited articles in this journal in 2007-2008.
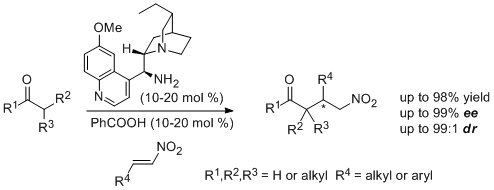
'Stereoselective synthesis of highly functionalized nitrocyclopropanes via organocatalytic conjugate addition to nitroalkenes'
S. H. McCooey, T. McCabe and S. J. Connon*, J. Org. Chem. 2006, 71, 7494.
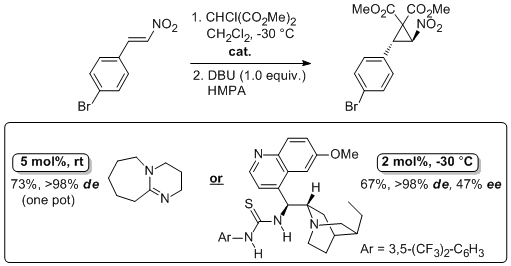
'Urea- and thiourea-substituted cinchona alkaloid derivatives as highly efficient bifunctional organocatalysts for the asymmetric addition of malonate to nitroalkenes: inversion of configuration at C9 dramatically improves catalyst performance'
S. H. McCooey and S. J. Connon*, Angew. Chem. Int. Ed. 2005, 44, 6367.
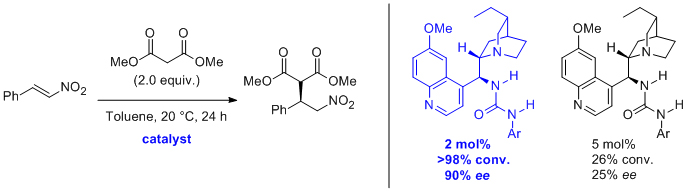
'Acrylamide in the Baylis-Hillman reaction: expanded reaction scope and the unexpected superiority of DABCO over more basic tertiary amine catalysts'
C. Faltin, E. M. Fleming and S. J. Connon*, J. Org. Chem. 2004, 69, 6496.
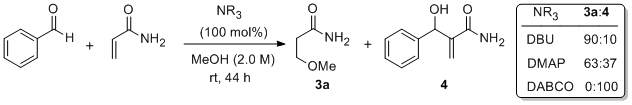
'Acceleration of the DABCO-promoted Baylis–Hillman reaction using a recoverable H-bonding organocatalyst'
D. J. Maher and S. J. Connon*, Tetrahedron Lett. 2004, 45, 1301.
Prize for being in the top 50 cited articles in this journal from 2004-2007.